HCP'S CORNER
about Mefenamic Acid
Read more about Mefenamic Acid's journey, mechanism of action and its efficiency

Mefenamic Acid
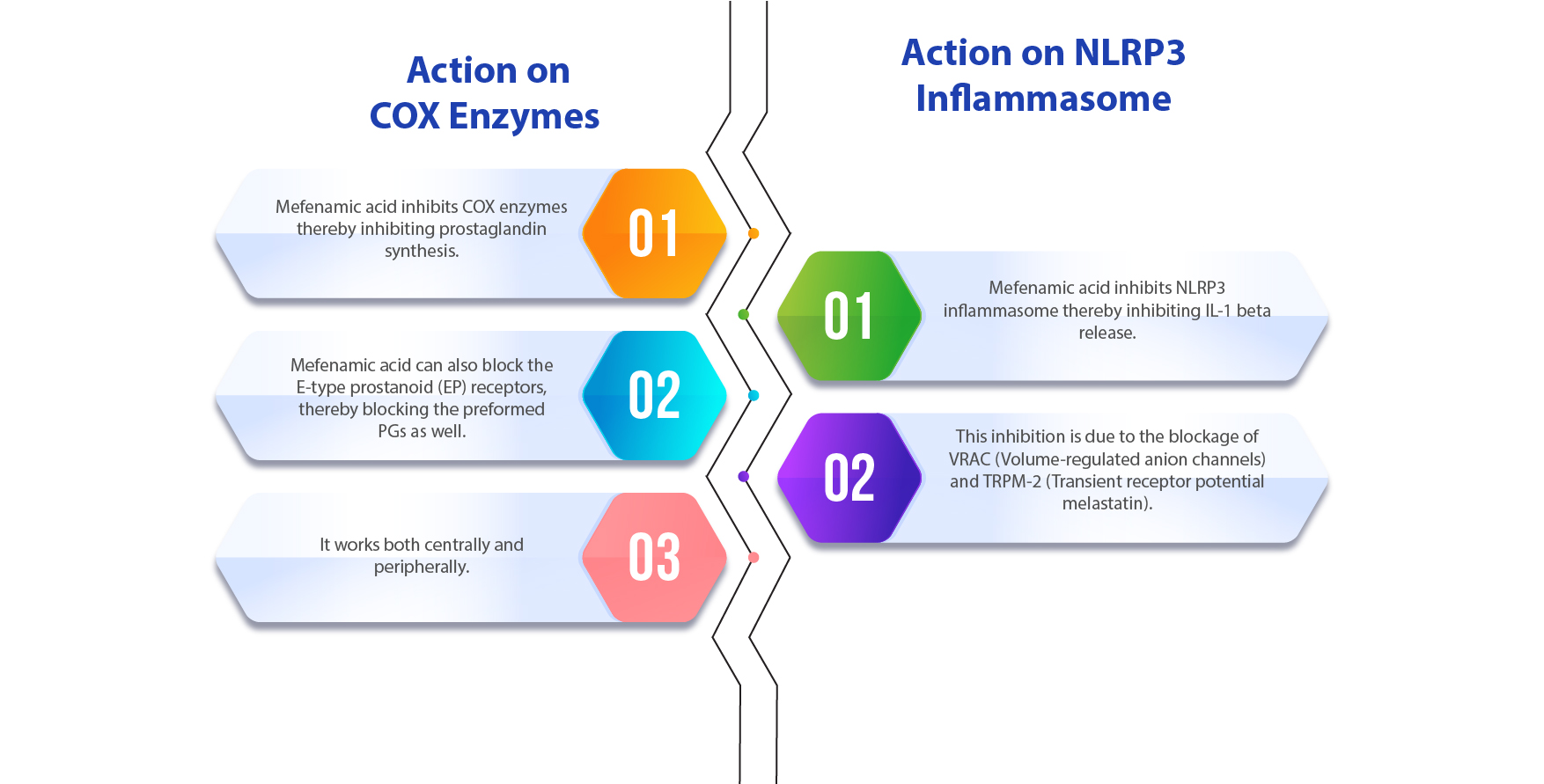
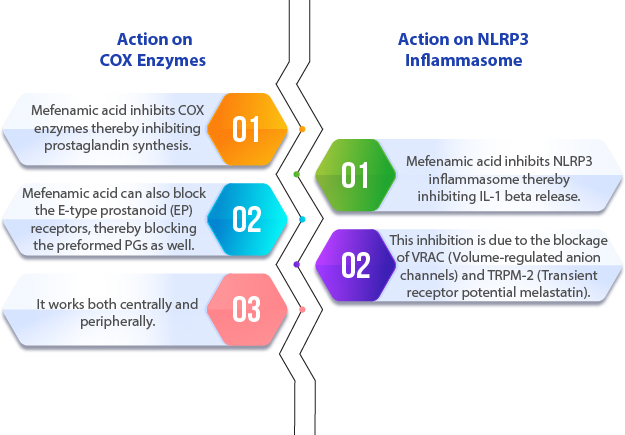

Mechanism of Mefenamic Acid

Uniqueness of Mefenamic Acid
Unique NSAID (Non-steroidal anti-inflammatory drugs) with a plethora of physiological effects, clinically helpful for its anti-pyretic, analgesic, and anti-inflammatory effects.

Efficacy and Safety
References
1. [Ref: Feng X, Wang X. Comparison of the efficacy and safety of non-steroidal anti-inflammatory drugs for patients with primary dysmenorrhea: A network meta-analysis. Mol Pain. 2018; 14:1744806918770320.]
2. .[Ref: Feng X, Wang X. Comparison of the efficacy and safety of non-steroidal anti-inflammatory drugs for patients with primary dysmenorrhea: A network meta-analysis. Mol Pain. 2018; 14:1744806918770320.]
3. [Ref: INTERNET. Mefenamic Acid. In LiverTox: Clinical and research information on drug-induced liver injury. January 10, 2020. Accessed on November 2, 2022, from https://www.ncbi.nlm.nih.gov/books/NBK548029/]
4. [Ref: Asif M. Am. J. Med. Stud. 2014; 2(1): 24-30, https://www.uktis.ora/www/medicinesinpregnancy.org]
Pharmacopeia references

Other Sections to view